Disclaimer:
This is a popular science article, which aims to popularize the relevant knowledge in the field and promote science communication.
The video, pictures, text and other materials used in this article, if involved in the copyright of works, please contact us in a timely manner, we will properly handle according to law.
In addition, if there are errors in the content of the article, or inconsistent with the original journal article point of view, conclusion, also welcome to inform us, we will be the first time to modify, delete and so on.
At the same time, we warmly welcome scientific researchers and science enthusiasts in related fields to contribute, recommend, or discuss cooperation to jointly promote the development of industrialization.
Osteosarcoma, a highly malignant tumor predominantly affecting adolescents, is characterized by high metastatic potential. Current limb salvage therapies (tumor resection +reconstruction + chemo/radiotherapy) face two critical challenges: chemo-radio-resistance leading to frequent recurrence, and a poor post-operative bone regeneration microenvironment. Although traditional 3D-printed bone scaffolds exhibit osteoconductivity, they fail to simultaneously achieve tumor elimination and bone regeneration. Recently, the team of Chong Cheng, Li Qiu, and Boqing Zhang from Sichuan University made a significant breakthrough by designing and developing a novel intelligent 3D-printed scaffold (HS-ICTO). This scaffold can intelligently switch from a "tumor-killing mode" to a "tissue-regeneration mode" in response to the tumor microenvironment and external
ultrasound stimulation, providing an innovative integrated solution for osteosarcoma treatment and post-operative bone defect repair. Relevant research was published in Nature Communications under the title "Sono-activable and biocatalytic 3D-printed scaffolds for intelligently sequential therapies in osteosarcoma eradication and defect regeneration"

Highlights: Innovation and Breakthroughs
(1)Intelligent Sequential Therapy: The scaffold automatically switches its function based on the pH of the microenvironment—achieving efficient tumor killing in the acidic tumor microenvironment (TME) and promoting regeneration in the neutral defect microenvironment—without additional regulation.
(2)Ultrasound Controllability: Ultrasound activation enables spatiotemporally precise enhancement of reactive oxygen species (ROS) generation, reducing toxic side effects on normal tissues and realizing "on-demand therapy".
(3)Multifunctional Integration: Simultaneously addresses three key issues—tumor recurrence, bone regeneration disorders, and imbalance—reducing the number of surgical procedures.
(4)Clinical Translation Potential: The 3D-printed hydroxyapatite (HA) scaffold (HS) has mechanical properties matching human trabecular bone, excellent biocompatibility, and high stability of ICTO nanoparticles, allowing long-term functional performance.
WHAT:Research Content
Aiming to reduce the need for multiple invasive procedures in osteosarcoma treatment, the team developed a novel sono-activable and biocatalytic 3D-printed HA scaffold (HS-ICTO) for intelligent sequential therapy in osteosarcoma eradication and bone defect regeneration. Modified with ICTO nanoparticles (Ir clusters anchored on TiO₂ nanosheets), the scaffold rapidly generates massive ROS via multienzyme-like (peroxidase/POD-, oxidase/OXD-like) mechanisms coupled with sono-activation in the TME, thereby enhancing tumor cell apoptosis. In the inflammatory bone defect microenvironment, it intelligently switches to exert catalase (CAT)-like activity—catalyzing hydrogen peroxide (H₂O₂) into oxygen (O₂)— which effectively blocks endogenous H₂O₂-mediated oxidative stress, modulates the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs), and ultimately facilitates bone defect regeneration.
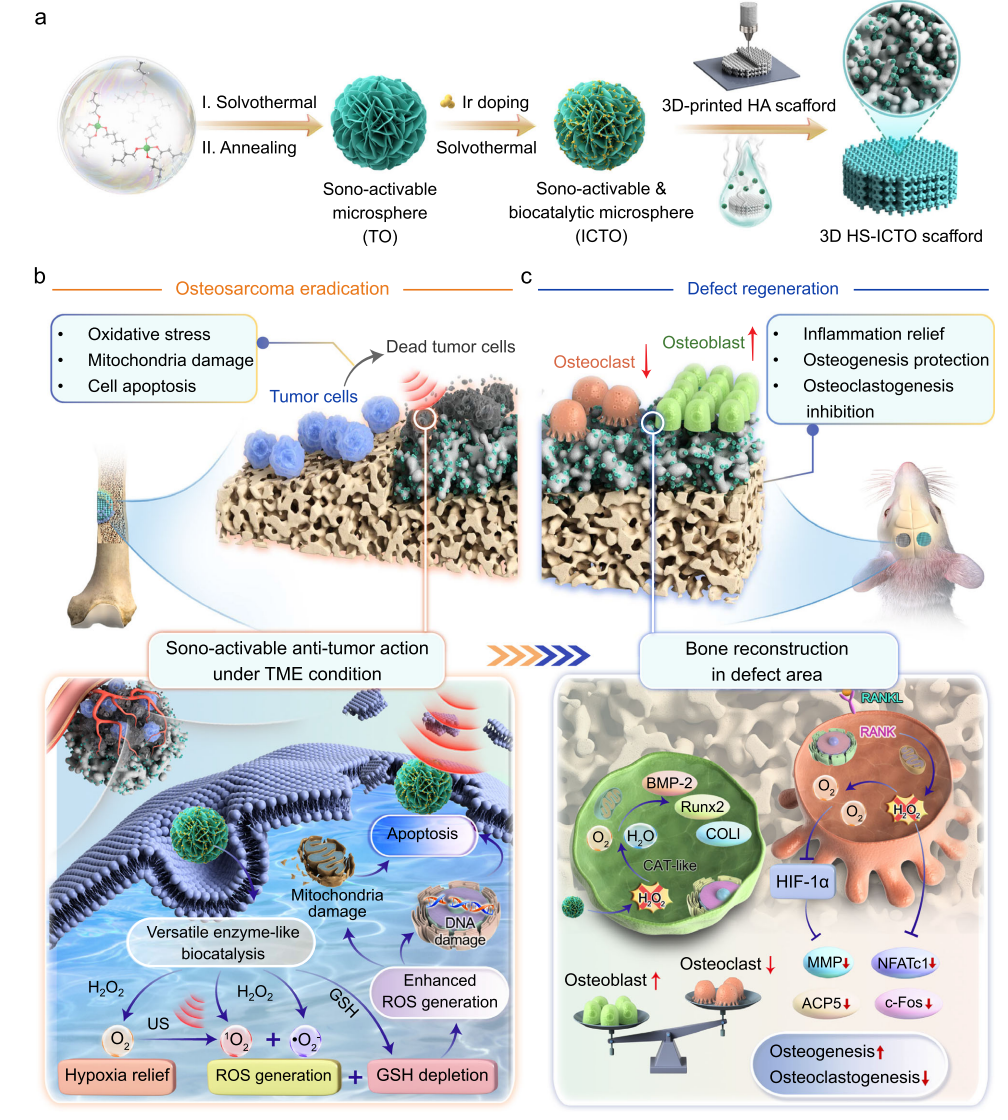
Fig. 1 | Design of the sono-activatable and enzyme-mimetic catalytic 3D-printed scaffold for limb salvage therapy of osteosarcoma.
WHY:Research Background and Significance
Osteosarcoma is a highly malignant bone tumor that predominantly occurs in children and adolescents, often invading long bones and leading to bone destruction, pain, and even metastasis. The current mainstream limb salvage therapy (tumor resection + bone defect reconstruction + radio/chemotherapy) faces two core dilemmas: firstly, tumor resistance to radio/chemotherapy makes radical resection difficult, leading to high local recurrence rates and often necessitating multiple surgeries; secondly, the postoperative bone defect microenvironment, plagued by inflammation and oxidative stress imbalance, has limited regenerative capacity, severely impacting patient prognosis.
Although existing 3D-printed bone scaffolds (e.g., hydroxyapatite, HA) possess osteoconductivity, they cannot simultaneously address tumor killing and bone regeneration, nor can they easily adapt to the complex dynamic changes in the microenvironment. Therefore, developing a multifunctional scaffold that can intelligently respond to both the tumor and bone defect microenvironments to achieve sequential therapy—"eradicate tumor first, regenerate bone next"—is key to breaking through the bottleneck in osteosarcoma treatment.
HOW:Research Methods
(1)Material Synthesis and Characterization: Petaloid TiO₂ (TO) was prepared via a solvothermal method. Iridium (Ir) was doped through hydrothermal treatment to form ICTO nanoparticles, which were then deposited onto the surface of a 3D-printed HA scaffold (HS) via a wet deposition method to obtain HS-ICTO. Material structure was validated using techniques such as SEM, TEM, XRD, and XPS. The electronic coupling effect of Ir-TiO₂ was analyzed via XANES and EXAFS.
(2)In Vitro Experiments: Enzyme activity assays: POD/OXD/CAT-like activities of ICTO and the ultrasound enhancement effect were verified through TMB colorimetric assays and EPR radical trapping. Cell experiments: The killing effect of HS-ICTO on osteosarcoma cells (143b) was evaluated (Live/Dead staining, flow cytometric apoptosis analysis), alongside its protective effect on the osteogenic differentiation of BMSCs (ALP and Alizarin Red staining, RT-qPCR).
(3)In Vivo Experiments: Tumor model: The anti-tumor effect of HS-ICTO combined with ultrasound was validated in a subcutaneous osteosarcoma model in nude mice (tumor volume, apoptosis marker detection). Bone regeneration model: Bone regeneration efficiency was assessed in a rat cranial defect model via Micro-CT and histological staining (bone volume, osteogenic marker expression)
(4)Mechanism Validation: Differential gene expression was analyzed using RNA-seq. The catalytic reaction pathway of ICTO was elucidated through DFT calculations.
Solution: Technical Advantages
1、Material Design and Structural Validation
The HS-ICTO was constructed based on a 3D-printed hydroxyapatite (HA) scaffold. ICTO nanoparticles (Ir-TiO₂ clusters) were modified onto the scaffold surface via wet deposition. The strong electronic coupling between Ir and TiO₂ endowed it with multifunctional catalytic activity. The figure below clearly illustrates the synthesis route of the scaffold and its "dual-stage action mechanism": In the TME, ICTO generates ROS via peroxidase (POD)- and oxidase (OXD)-like activities combined with ultrasound activation to kill tumors; in the bone defect microenvironment, it switches to catalase (CAT)-like activity, decomposing H₂O₂ to generate O₂ and promote bone regeneration. SEM and TEM confirmed that Ir clusters in ICTO, with an average size of ~1.7 nm, were uniformly distributed on the TiO₂ surface. XPS analysis revealed Ir-O-Ti electronic coupling, ensuring catalytic stability. The scaffold's mechanical properties were comparable to human trabecular bone, meeting implantation requirements.
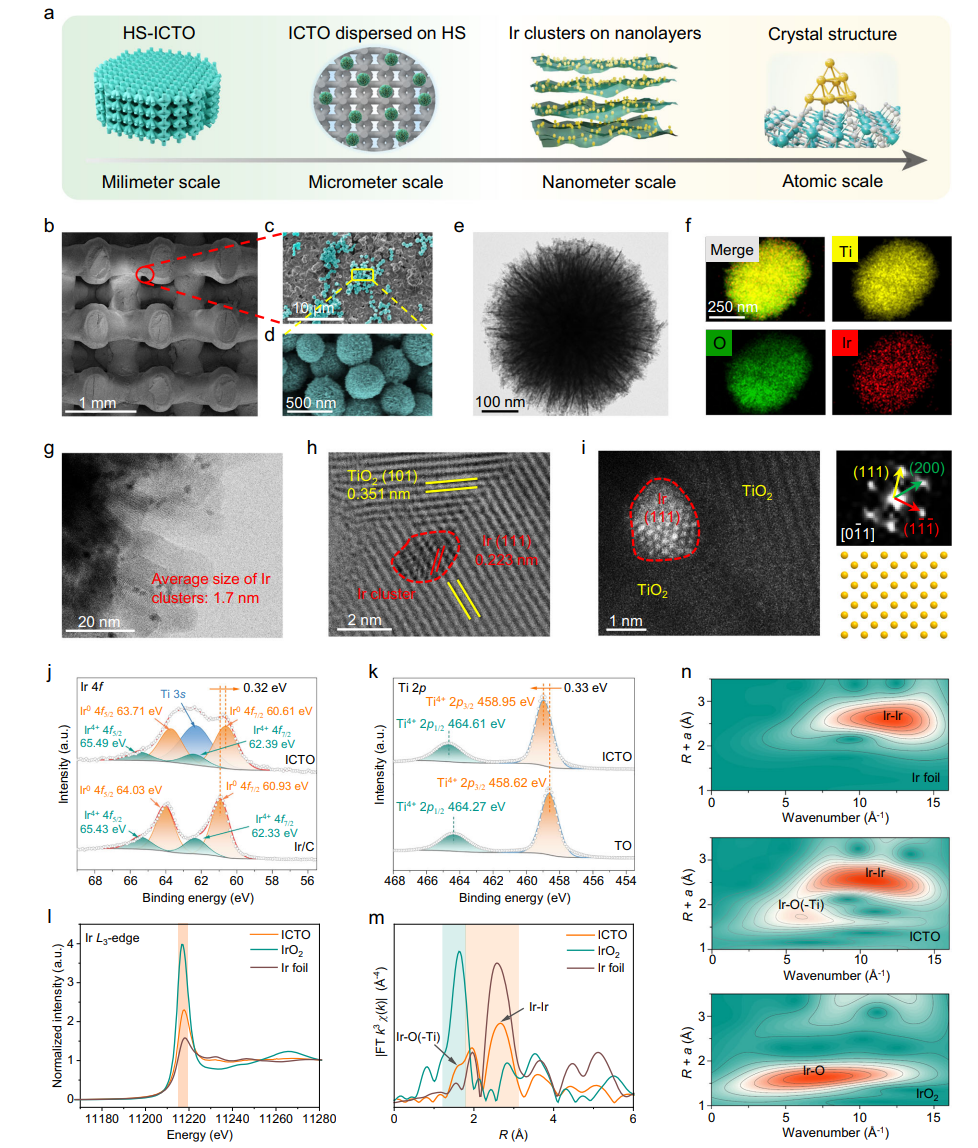
Fig. 2 | Morphology and structural characterization of the HS-ICTO scaffold.
2、Enzyme-like Activities and Catalytic Mechanism
The enzyme-like activities of ICTO in different microenvironments were verified, revealing its switching mechanism between "ROS generation" and "oxygen release." In the acidic TME, the POD/OXD-like activity of ICTO was significantly higher than that of pure TiO₂, efficiently generating ROS as shown by the TMB colorimetric reaction. In the neutral bone defect environment, its CAT-like activity decomposed 90% of H₂O₂ within 20 minutes while continuously producing oxygen. DFT calculations elucidated the catalytic pathways: under acidic conditions,・OOH is generated via Ir-Ti synergistic sites (energy barrier 0.51 eV); under neutral conditions, O₂ is generated (energy barriers 0.34-0.38 eV), confirming microenvironment-responsive specificity.
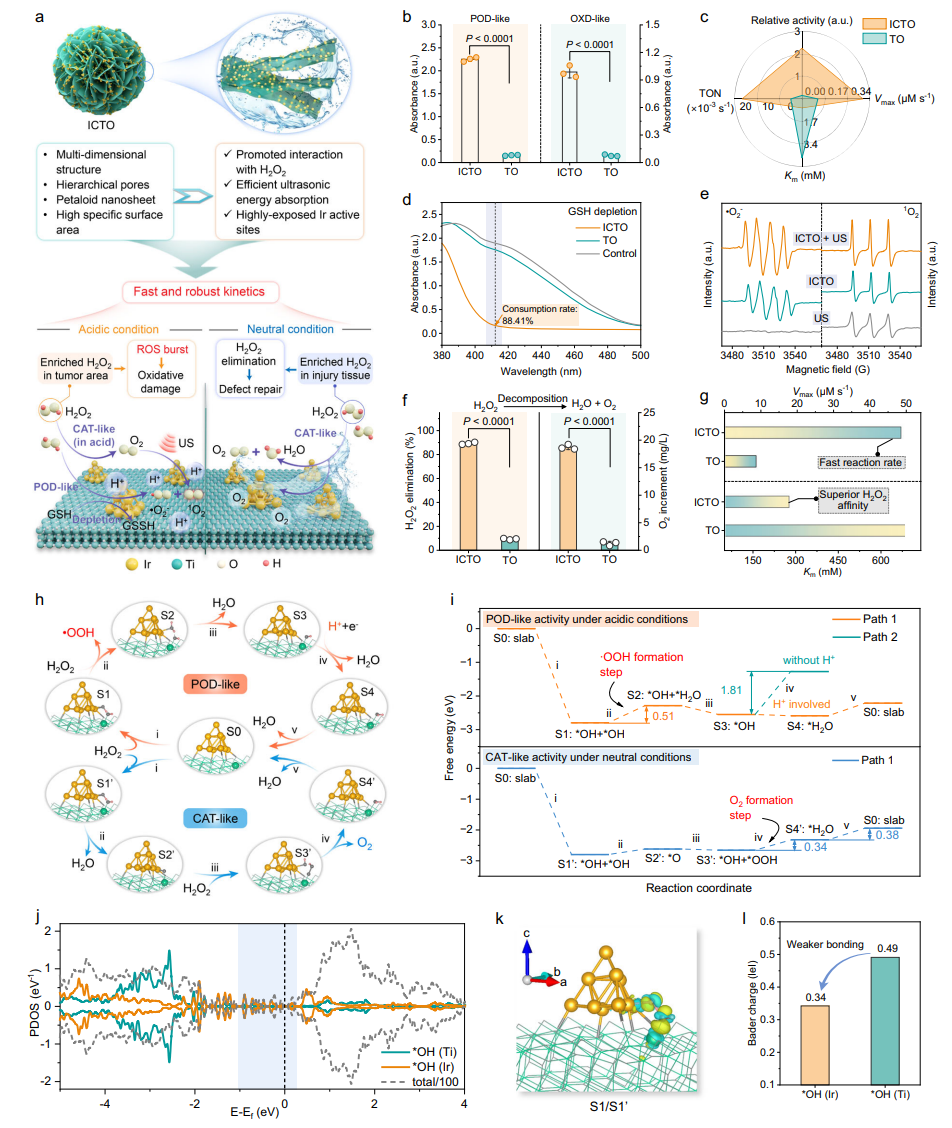
Fig. 3 | Enzyme-mimetic catalytic activities and theoretical calculations of ICTO
3、Tumor-killing Efficacy
The killing efficiency of HS-ICTO against osteosarcoma, both in vitro and in vivo, and its synergistic effect with ultrasound were evaluated. In vitro experiments showed an apoptosis rate of 87.13% for 143b osteosarcoma cells in the HS-ICTO + US group. RNA-seq confirmed this was achieved by upregulating genes related to apoptosis and ROS damage, while also reducing HIF-1α expression to alleviate tumor hypoxia. In the in vivo nude mouse model, the HS-ICTO + US group exhibited a tumor inhibition rate of 90.43%. H&E and TUNEL staining revealed extensive apoptosis in tumor tissue, and the scaffold showed no significant toxicity.
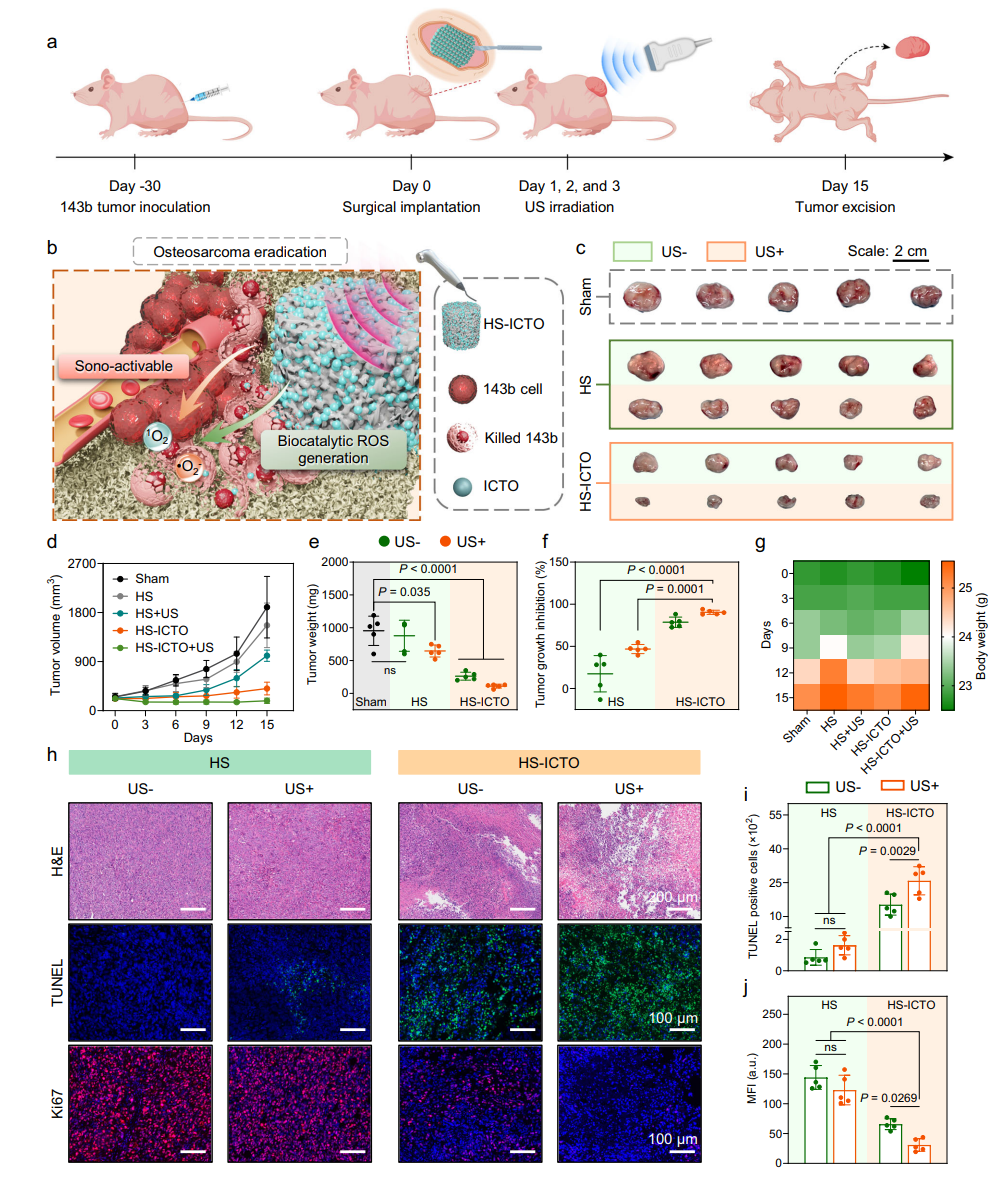
Fig. 4 | In vivo therapeutic effects of HS-ICTO in an osteosarcoma xenograft model
4、Bone Regeneration and Microenvironment Modulation
The study validated the protective effects of HS-ICTO on stem cells, its promotion of osteogenic differentiation, and its inhibition of osteoclastogenesis. Stem Cell Protection: HS-ICTO resisted H₂O₂-induced damage, maintained the viability of bone marrow mesenchymal stem cells (BMSCs), significantly enhanced osteogenic capability as shown by ALP and Alizarin Red staining, and upregulated the expression of osteogenic genes such as Runx2 and BMP-2.
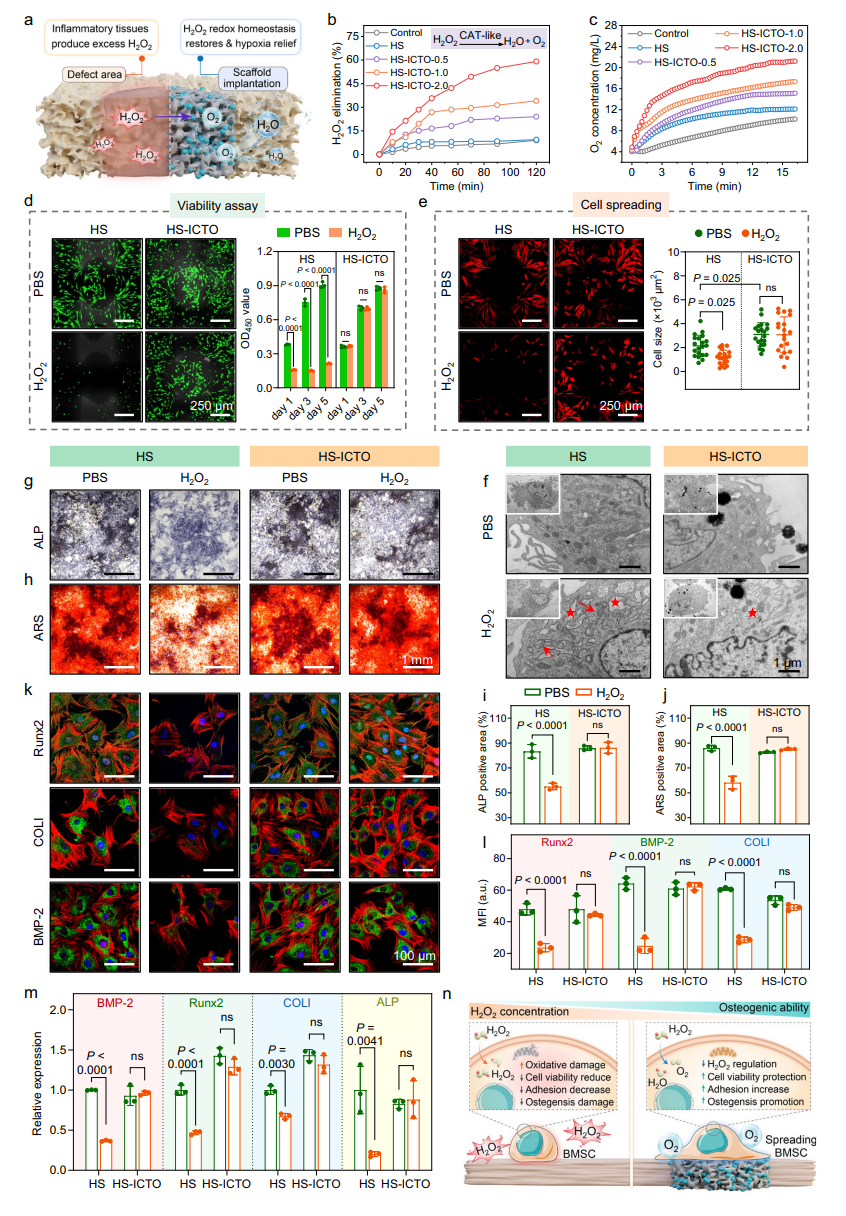
Fig. 5 | HS-ICTO modulates redox homeostasis and protects stem cells from oxidative stress damage in vitro.
Osteoclast Inhibition: By suppressing the expression of genes like NFATc1 and MMP-9, HS-ICTO reduced osteoclast activity and blocked bone resorption. TRAP staining showed a significant decrease in the number of positive cells.
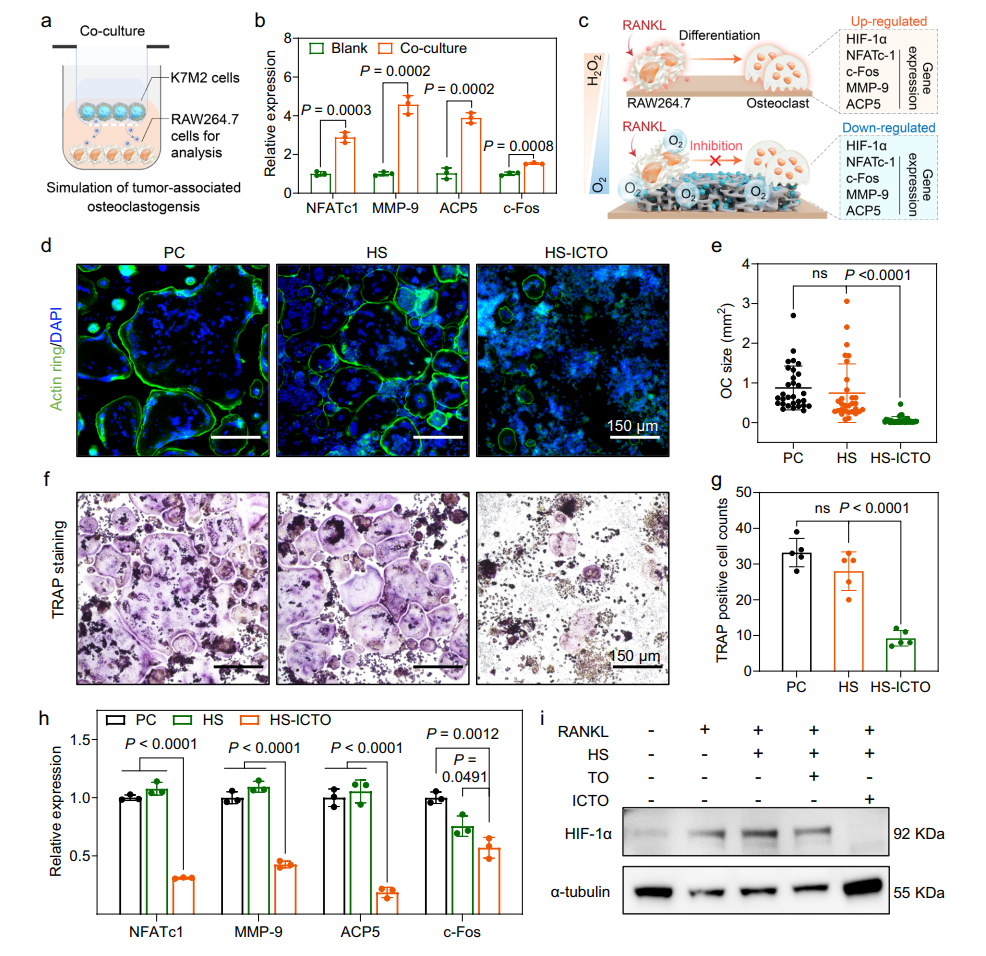
Fig. 6 | Inhibitory effect of HS-ICTO on osteoclastogenesis.
Bone Regeneration: In the rat cranial defect model, the bone volume/tissue volume (BV/TV) ratio in the HS-ICTO group reached 43.6% at 12 weeks (significantly higher than the 28.7% in the HA group, Fig. 8h). Enhanced expression of osteogenic markers (Runx2, BMP-2) confirmed efficient bone repair capability.
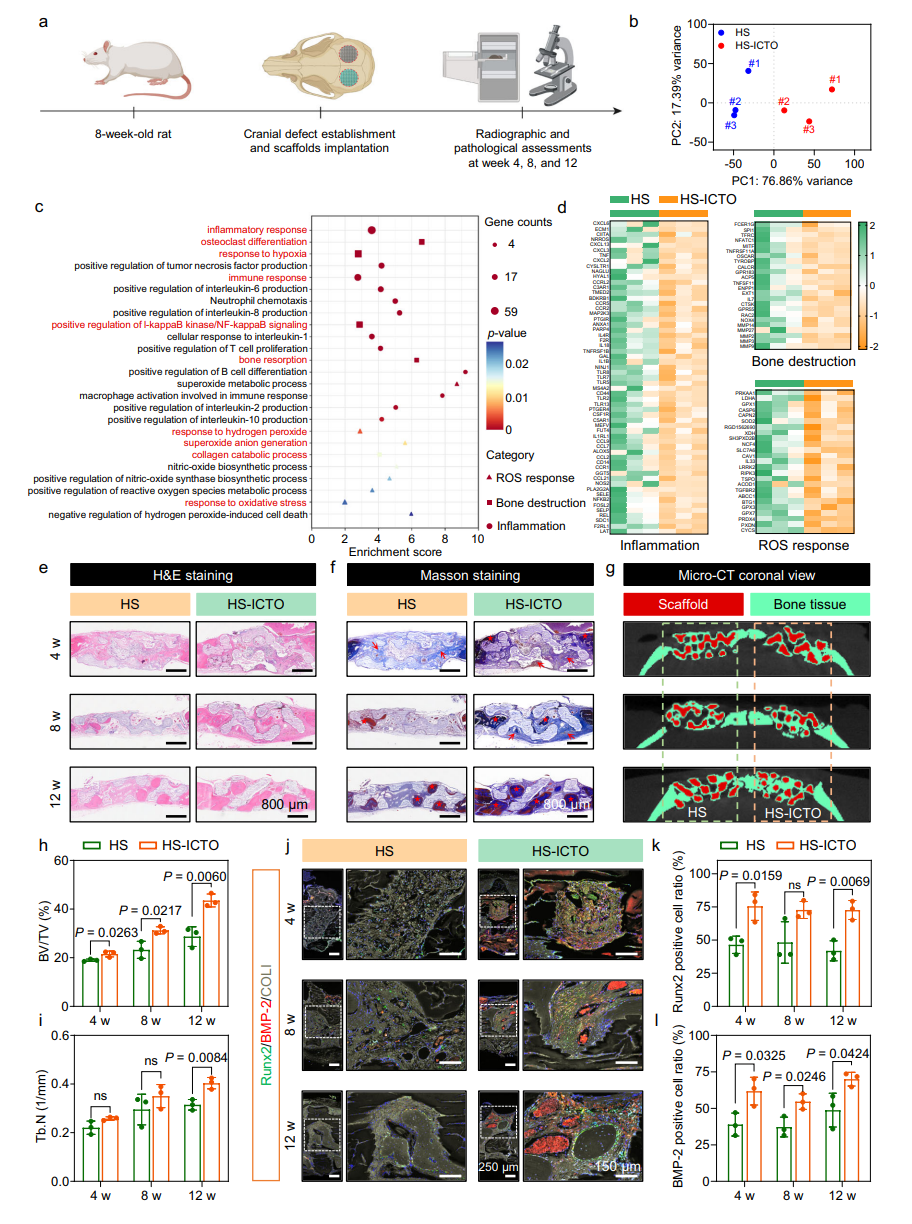
Fig. 7 | In vivo bone regeneration assessment in a rat cranial defect model.
Conclusion
To address the limitations of current osteosarcoma limb salvage therapies, the team developed a sono-activable and biocatalytic 3D-printed HA scaffold (HS-ICTO) that realizes intelligent sequential therapy of "tumor killing → bone regeneration". By leveraging the microenvironment-responsive enzyme activity of ICTO and ultrasound controllability, HS-ICTO efficiently eliminates osteosarcoma cells (via ROS generation) and promotes bone defect repair (via H₂O₂ scavenging/O₂ supply), while exhibiting excellent biocompatibility and mechanical stability. This work provides a promising strategy for precision oncological intervention and regenerative medicine, offering new hope for reducing surgical procedures and improving prognosis in osteosarcoma patients.

