Statement:
This is a popular science article, which aims to popularize the relevant knowledge in the field and promote science communication. The video, pictures, text and other materials used in this article, if involved in the copyright of works, please contact us in a timely manner, we will properly handle according to law. In addition, if there are errors in the content of the article, or inconsistent with the views and conclusions of the original journal article, you are also welcome to inform us at any time. We will modify and delete the article at the first time. At the same time, we warmly welcome scientific researchers and science enthusiasts in related fields to contribute or recommend articles to us, or discuss cooperation to jointly promote the development of industrialization
Li Dichen and He Jiankang teams at Xi 'an Jiaotong University have developed a new type of electrically printed InterPore microfiber lattice.
The study aims to recreate the structural and functional characteristics of myocardial tissue in vitro by promoting high-density cell alignment and enhancing tissue connectivity through anisotropic fibrin remodeling in 3D-printed microfiber lattices. The related work was published in 《Advanced Materials》 under the title "Engineering Highly Aligned and Densely Populated Cardiac Muscle Bundles via Fibrin Remodeling in 3D-Printed Anisotropic Microfiber Lattices".

Key Highlights: Innovation and Breakthroughs
(1) Design and Fabrication of InterPore Microfiber Lattice: Utilizing electrohydrodynamic (EHD) printing technology, we developed an anisotropic InterPore microfiber lattice. The lattice consists of longitudinal and transverse microfiber walls arranged in a staggered "V-shaped" configuration, which significantly enhances vertical connectivity between pores.
(2) Cell Arrangement and Tissue Formation: The InterPore lattice guides cells to align along the longitudinal axis, forming continuous, high-density cell clusters. Within this lattice structure, these clusters develop faster, demonstrate higher survival rates, and maintain tissue integrity and functionality even under high-density conditions.
(3) Electrophysiological properties of cardiac tissue: Cardiac tissue in the InterPore lattice exhibits synchronized calcium wave propagation, indicating enhanced electrical coupling and synchronous contraction capability. Under electrical stimulation, cardiac groups in the InterPore lattice
WHAT: Research Content
This study addresses the challenges of disordered cell arrangement and electrical signal conduction blockage in myocardial tissue engineering by developing a 3D-printed anisotropic microfiber lattice scaffold. The scaffold integrates dual functional structures: vertically interconnected channels achieve high-density cell alignment through "V-shaped intersecting transverse fibers" to guide continuous myocardial bundle assembly; anisotropic topology regulates fibrin dynamic remodeling via mechanical constraints, promoting synchronized intercellular electrical coupling. The hierarchical fiber network ensures mechanical stability and nutrient permeability, achieving spatiotemporal synergy between structural guidance and cellular self-assembly. Validation through in vitro cell experiments, electrophysiological characterization, and animal models demonstrates that this scaffold significantly enhances myocardial tissue contraction synchronization rate and calcium signal transduction efficiency, providing a highly biomimetic engineering platform for cardiac disease treatment and drug screening.
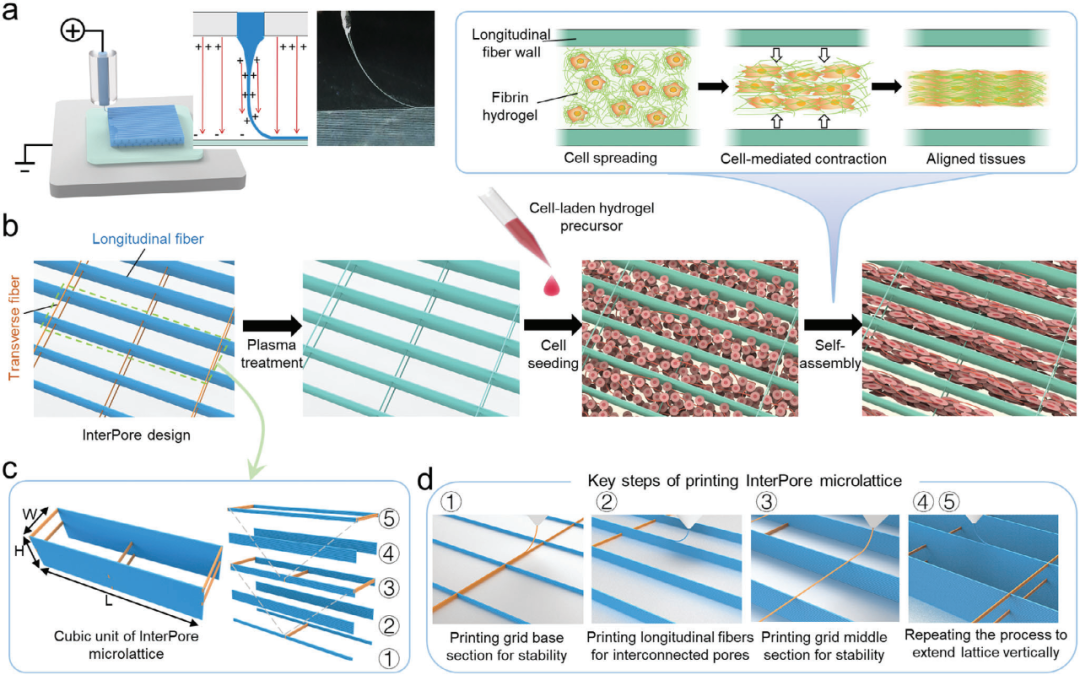
Figure 1 Design and preparation of InterPore microporous lattice
WHY: Research Background and Significance
The functionality of cardiac tissue relies on the precise alignment and synchronized electrical signal conduction of cardiomyocytes (CMs)to maintain efficient contraction and blood pumping. However, pathological conditions such as myocardial infarction disrupt this structural integrity, leading to functional impairment. Traditional myocardial tissue engineering methods (e.g., electrospun scaffolds or hydrogel systems) exhibit limitations: inadequate cell alignment, impaired electrical signal transmission, poor mechanical stability, and difficulty achieving high cell density (approaching 108-109 cells/cm³ in natural myocardium). This study develops an anisotropic 3D-printed scaffold that guides cell-mediated fibrin remodeling to form highly aligned and dense continuous myocardial bundles. This approach achieves spatiotemporal coordinated cell arrangement and optimized electrophysiological functions, providing a novel platform for cardiac repair.
HOW: Research Methods
1. Scaffold Fabrication: Medical-grade polycaprolactone (PCL) was used as the core scaffold material. Anisotropic microfiber lattice scaffolds were manufactured stepwise through electrowhydraulic (EHD) printing technology. The innovative "V-shaped interlaced transverse fibers" were introduced, and the scaffold was loaded with fibrin hydrogel after plasma treatment.
2. Scaffold characterization: The microstructural morphology and architecture were characterized through SEM, micro-CT 3D reconstruction, and mechanical property testing. The scaffold's myocardial cell repair efficacy was evaluated using cell-mediated remodeling tracking and electrophysiological validation.
3. Multiscale performance verification: Functional assessments of engineered cardiac tissue were conducted through immunofluorescence staining, gene temporal expression analysis, calcium transient analysis, and rhythm rate evaluation. Pharmacological sensitivity validation demonstrated its intelligent responsiveness to drug delivery, while multiple seeding experiments and finite element modeling were employed to assess its vascularization potential.
Solution: Technical Advantages
1. InterPore Microfiber Lattice for Developing Newborn Rat Cardiac Tissue
By culturing neonatal rat cardiomyocytes in fibrin hydrogel and cultivating them in different lattice structures, this study investigated the promoting effect of InterPore microfiber lattices on cardiac tissue development. The results demonstrated that the cardiac tissues cultivated in these lattices exhibited more mature structural characteristics and electrophysiological activity.
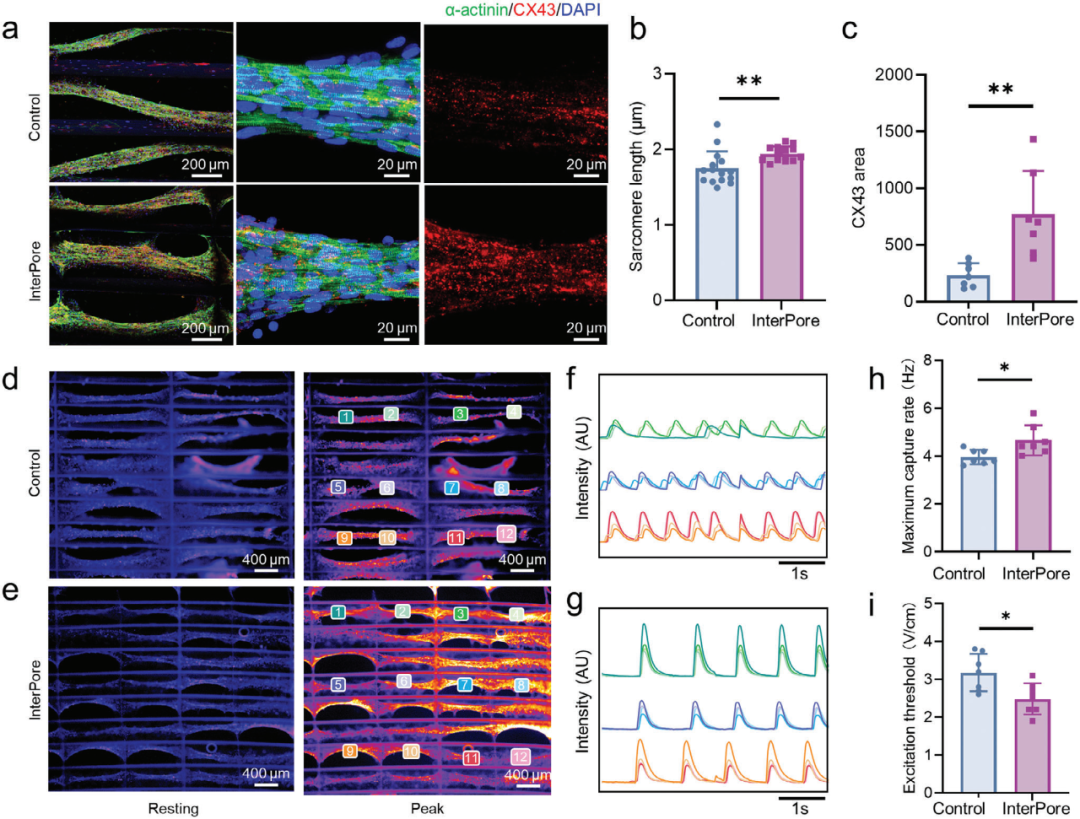
Figure 2. Structural characterization and electrophysiological function of the engineered arranged cardiac tissue
2. Multiple sowing cycles enhance cell load and multicellular tissue formation
Using multiple sowing experiments and finite element simulation, the effects of multiple sowing cycles on cell load and multicellular tissue formation in InterPore microlattice were investigated. The results showed that multiple sowing cycles could improve cell density, maintain high cell viability, and construct prevascularized cardiac tissue.
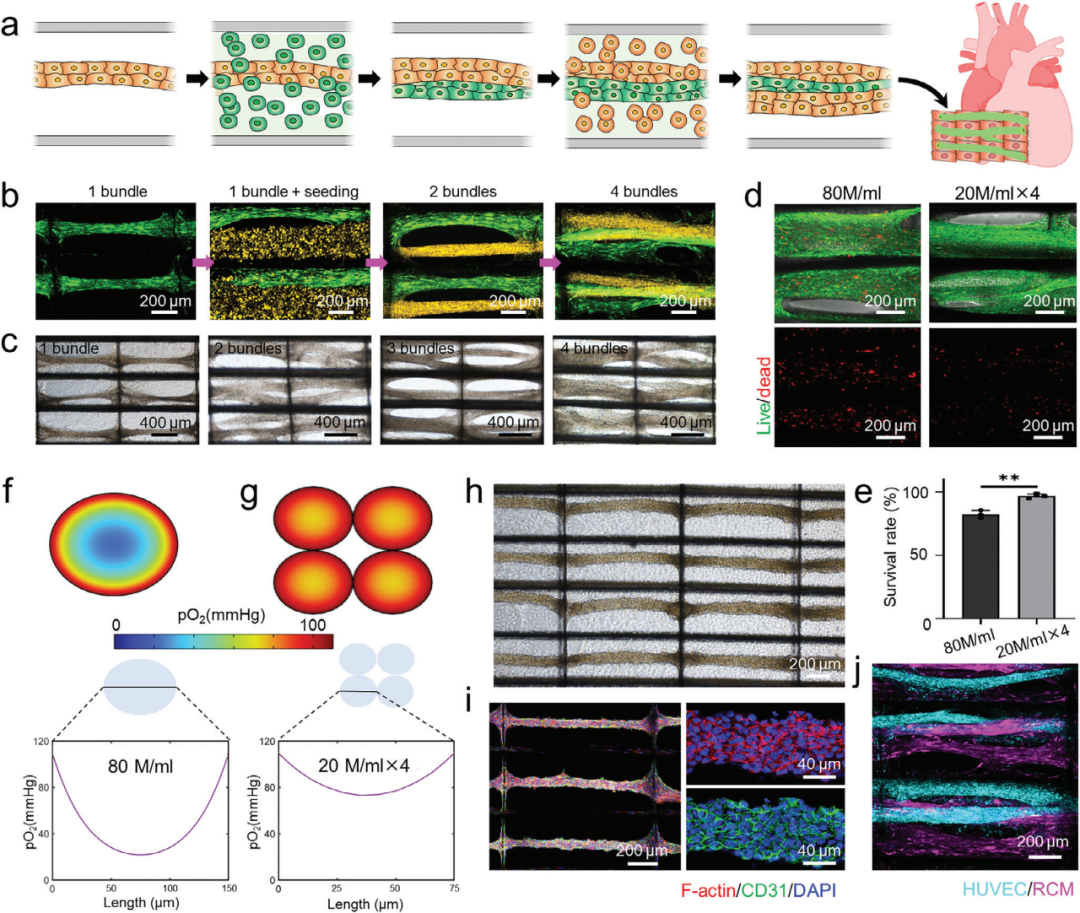
Figure 3. Multiple seeding cycles promote enhanced cell load and multicellular tissue engineering in InterPore microfiber lattice
3. To construct functional human cardiac tissue using hiPSC-CMs
Methods such as cell culture, calcium imaging, gene expression analysis and drug treatment were used to study the process of constructing functional human heart tissue using hiPSC-CMs in InterPore microfiber lattice. The results demonstrated that the InterPore microfiber lattice effectively promotes functional maturation of hiPSC-CMs.The developed tissue exhibited drug responsiveness and can be utilized for pharmaceutical testing.
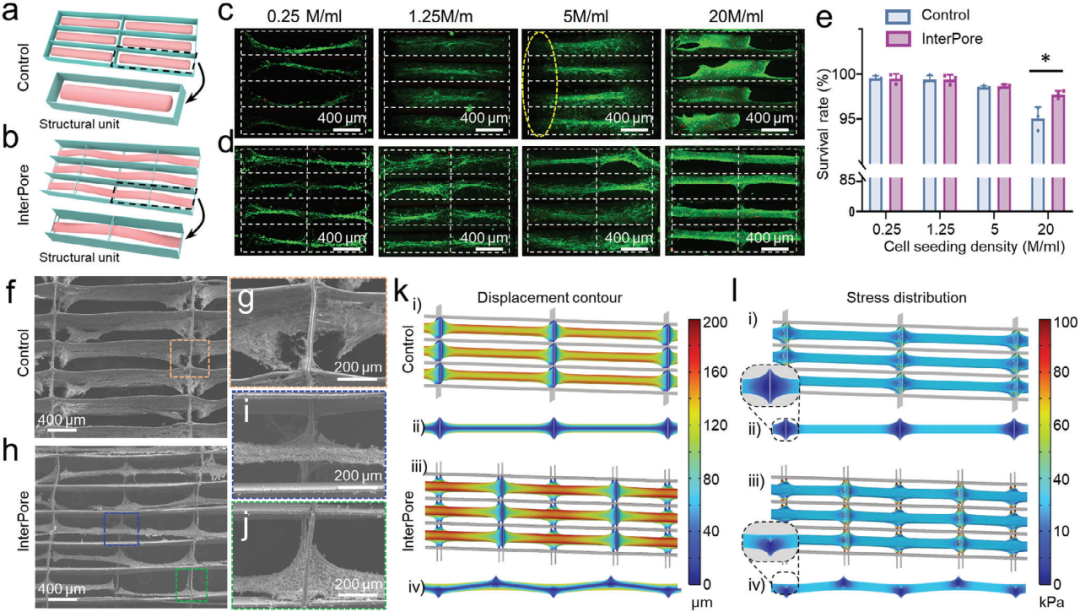
Figure 4. Characterization of engineered arrangements of human cardiac tissue constructed from hiPSC-CMs in the InterPore microfiber lattice
Conclusion
The study developed an anisotropic 3d-printed InterPore fiber lattice for repair of cardiac myocytes. By integrating precise structural control with bio-inspired fibrin hydrogel remodeling, this platform achieves highly aligned and dense myocardial bundles with enhanced electrophysiological and mechanical properties. The longitudinally interconnected pores of the lattice not only promote high-density cellular organization and robust mutual connectivity, but also support key processes such as angiogenesis and functional tissue maturation. Lookingforward to the future , this approach has great potential for advancing applications in disease modeling, drug screening, and regenerative therapies to provide a scalable, highly biomimetic technology platform for cardiac tissue engineering.

